440innovations, llc has provided innovative, practical, & efficient solutions for healthcare & surgeons since 2011.
We Provide Innovation...

Federal Supply Schedule, FSS, 36F79721D0199
FDA Operator Number 10081154
Mission

ONE
To provide surgeons and allied health professionals with innovative and pragmatic solutions.
TWO
As a Veteran Owned Small Business, we will maintain the highest level of quality, integrity, and respect for our suppliers, clients, and patients.


THREE
We will work with Business Partners & Sub-Contractors that are known experts in their respective fields.
440innovations Goal
Through the course of living there are many opportunities for trauma, disease, or degenerative changes to disrupt our balance of life. We attempt to assist healthcare providers and surgeons in procuring the most advanced solutions for individual patient care and rehabilitation.
At 440innovations our goal is to help patients reestablish balance to an interrupted life and reach their full God given potential.
"There are only two ways to live your life. One is as thought nothing is a miracle. The other is as though everything is a miracle... Only a life lived for others is worth living." Albert Einstein
"Stretch out your hand to heal and perform signs and wonders through the name of your holy servant Jesus.” Acts 4:30
Companies Represented & Territory
Clicking on the Company Logo will exit this website.
Biologic
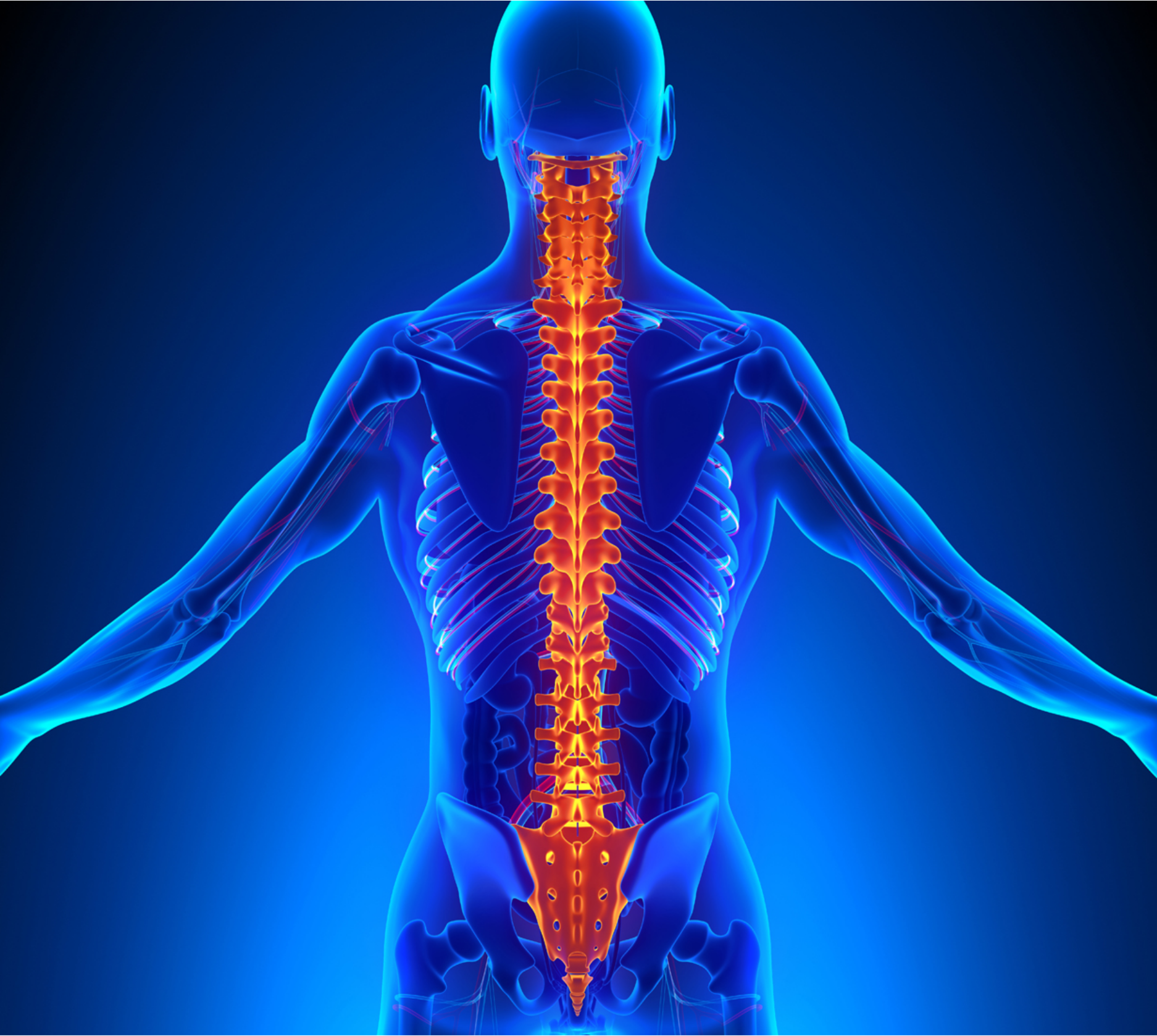
Spine
Aesculap
Altrux Associate /Lumbar Total Disc Replacement -Intelligent Motion Technology
activL® Artificial Disc
Hot & Cold Therapy
ThermaZone Thermal Therapy
Virginia, Kentucky, Oklahoma, Tennessee
Federal Supply Schedule
Contract Number V797D50436

Gowns, OR Drapes, Scope Drapes, Equine Drapes, Misc OR products
PO Box 12185, Newport News VA 23612-2185
Info@440innovations.com
+001.757.848.3354
Email us for more detailed information.