Augmented Reality / Artificial Intelligence
New AI and Augmented Reality TKA Platform Cleared by FDA
Orthopedics This Week, August 14th, 2020
By Elizabeth Hofheinz, M.P.H., M.Ed.
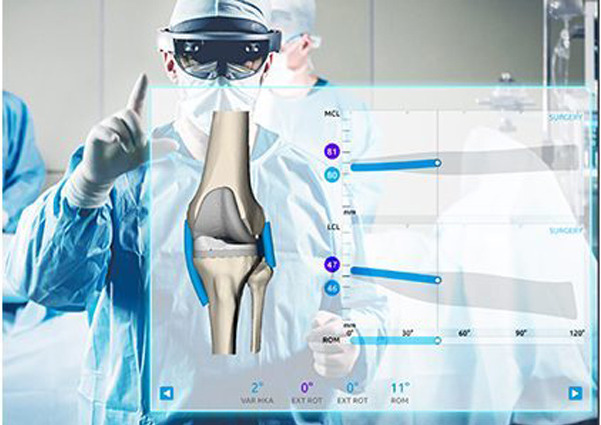
NOTE:
Shoulder, Total Knee Arthroplasty, & Spine Applications are FDA Cleared.
Sports Medicine and Hip TA are FDA Pending.
Courtesy of Medacta International S.A.
The U.S. Food and Drug Administration has cleared an augmented reality, artificial intelligence (AI) surgical platform for total knee arthroplasty. The manufacturer is Medacta International S.A., based in Castel San Pietro, Switzerland.
Medacta explained to OTW that the system, named NextAR TKA, which will eventually be extended to hip, shoulder and spine procedures, has been designed and engineered with artificial intelligence and machine learning that enhance the efficiency and precision of pre-operative CT-based planning and analysis.
According to Medacta, The NextAR TKA Application “…delivers personalized planning, with low upfront capital investment required by clinics and hospitals, as well as economic benefits to the healthcare system through OR efficiency and low cost per procedure…”
“With enhanced visualization via augmented reality, the NextAR Platform is an innovative and efficient tool for ultra-precise, patient-specific treatment,” said Dr. Michael McAuliffe (MBBS FRACS), an orthopedic surgeon at St Andrew’s Ipswich Hospital in Queensland, Australia, and a member of the expert surgeon panel that Medacta collaborated with to develop the NextAR TKA Application.
“With the NextAR TKA Application, we are able to track 3D soft tissue behavior during a surgery in real time. This functionality is an exciting advancement from traditional computer-assisted or robotic-assisted surgical systems that only track the relative movement of the knee bones, not the actual soft tissue.”
The NextAR TKA Application offers a reconstruction of the patient bone morphology and allows direct tracking of the collateral ligaments and a 3D analysis of soft tissue behavior throughout the whole range of motion during surgery. The company has also created the the NextAR TS, an infrared, single-use tracking system meant to improve efficiency and help surgeons execute preoperative plans.
“We are proud to be part of the NextAR Platform project that represents the next step forward for personalized medicine in orthopaedics,” said PD Dr. med. Sandro Fucentese, Head of Knee Surgery at the Balgrist University Hospital in Zurich and collaborator on this platform. “For many years we have been collaborating with Medacta in developing personalized solutions that have been relevant at the global level. The NextAR Platform perfectly fits into this philosophy relying on a solid and established ecosystem and expertise.”
Reflecting on a memorable moment, Francesco Siccardi, CEO of Medacta, told OTW, “The first time we showed the NextAR application to a small group of surgeons everybody wanted to experience it. The smile on their faces as they processed the personalized surgical information provided with the NextAR glasses was the best feedback we could get. Every single surgeon who tried the NextAR asked for a picture with glasses on!”
Asked where they hope to be one year from now, Siccardi noted, “We have designed NextAR technology to answer the current market needs for a technology platform able to address Medacta’s personalized medicine focus. We understand the efficiency and sustainability that new technology must provide in order to impact today’s clinical demands. One year from now, we will have the NextAR fully released in many key global markets. Specifically in the United States, we believe NextAR will make a significant impact in Ambulatory Surgical Centers, as we’ve virtually eliminated any concerns there may be for additional capital expense.”